Background: Persistent thrombocytopenia following allogeneic stem cell transplantation (alloHSCT) leads to an increase in morbidity and mortality. Causes of severe thrombocytopenia following HSCT include poor graft function, graft-versus-host disease (GVHD), infections, drug toxicities, underlying autoimmune diseases and relapse of underlying disease. Treatments can include thrombopoietin receptor agonists (TPO-RA), immunomodulators, and stem cell boost. Avatrombopag is a second generation TPO-RA approved for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients with poor response to standard treatments or in patients with chronic liver disease (CLD) associated thrombocytopenia requiring surgical interventions. Phase III clinical trials have shown greater platelet responses in chronic ITP and reduced transfusion needs in patients with CLD. Avatrombopag differs from other TPO-RA medications due to majority of elimination occurring in the gut, thus avoiding hepatic toxicity. Additionally, it does not require dietary restrictions. As there is limited literature in pediatric patients following alloHSCT, we describe the use of avatrombopag in a pediatric patient with chronic severe thrombocytopenia (<10 x10 3/uL) post HSCT.
Objective: To describe the use of avatrombopag for severe refractory thrombocytopenia in a pediatric alloHSCT recipient.
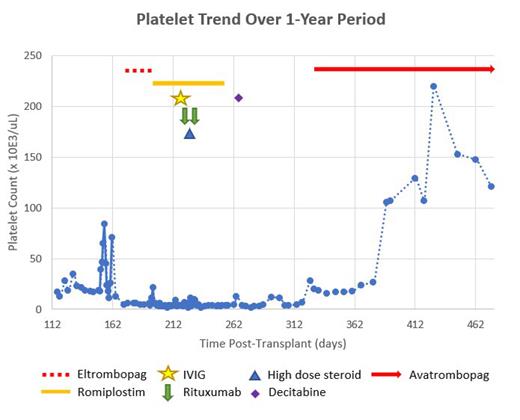
Case Presentation: We present a 14-year-old Hispanic male with Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) who received a maternal haploidentical HSCT with myeloablative conditioning and post-transplant cyclophosphamide. His post HSCT course was complicated by adenoviremia, hepatic variant GVHD, Enterobacter cloacae bacteremia and poor graft function. Seven months following HSCT, patient was found to be severely pancytopenic. Bone marrow morphology demonstrated 20-30% cellularity without evidence of leukemia relapse or GVHD. Lab evaluation was not consistent with transplant associated thrombotic microangiopathy. He received a stem cell boost for presumed poor graft function. Patient demonstrated partial response by resolution of neutropenia and anemia; however, severe thrombocytopenia persisted. Multiple bone marrow evaluations remained negative for disease with full donor chimerism and negative TCR clonality. CXCL9 was noted to be elevated at 4270 and Immature Platelet fraction was elevated at 8.8. Platelet antibodies remained negative and all findings consistent with persistent severe ITP. Multiple treatments including romiplostim; eltrombopag; high dose immune globulin (IVIG); rituximab; methylprednisolone; and decitabine were not successful in improving platelet count (Figure 1). Evaluation for severe thrombocytopenia remained negative. During the course of severe thrombocytopenia, patient required several hospitalizations for symptomatic thrombocytopenia, requiring multiple platelet transfusions. Due to the severity of disease, he was granted a compassionate use investigational new drug (IND 165837) for avatrombopag and began on day +332. Dosing was initiated at 20 mg daily with a dosage adjustment to achieve platelet recovery to >50 x10 3/uL free from transfusion. The dose was titrated per manufacturer to maximum dose of 40 mg daily with response to platelet count >100 x10 3/uL in approximately 6 weeks time. By three months of treatment, dose was titrated down 40 mg three times a week and 20 mg on alternating days. Patient continues to maintain platelets above 100 x10 3/uL with no medication toxicities or adverse events noted. Patient has been able to return to his pre-alloHSCT lifestyle including contact sports and re-immunizations.
OffLabel Disclosure:
Ligon:Amgen: Research Funding.
Avatrombopag is a second generation TPO-RA approved for the treatment of primary chronic immune thrombocytopenia (ITP) in adult patients with poor response to standard treatments or in patients with chronic liver disease (CLD) associated thrombocytopenia requiring surgical interventions.